Tech
Unlocking Cancer’s Molecular Processes | New York Tech

New research, led by a medical student and a cancer biologist at the College of Osteopathic Medicine (NYITCOM), aims to continue the historic work of a world-renowned Nobel laureate and explain why some human cells become cancerous, spread, and resist treatment.
Pictured from left: Manny Singh and Dong Zhang, Ph.D.
Our bodies depend on a tightly controlled and well-executed process of cellular reproduction to create and maintain healthy tissues and organs. When cells reproduce, the genetic materials (DNA) inside them are duplicated and passed on to new cells. However, for duplication to occur precisely and correctly, the cell’s “replication machinery” must overcome structural or molecular roadblocks in the process. The ends of chromosomes are genomic regions full of roadblocks, and failure to overcome these blocks can lead to damage, preventing proper duplication.
When damaged chromosome ends fuse with other broken chromosome ends, this initiates a cycle in which chromosomes break, fuse, form a bridge, and break again. Known as the “breakage-fusion-bridge (BFB) cycle,” this series of events causes a cascade of DNA mutations that eventually leads to tumor formation, metastasis (spreading), and drug resistance. The scars of the BFB cycle have been found in pancreatic cancer, osteosarcoma, and other cancers.
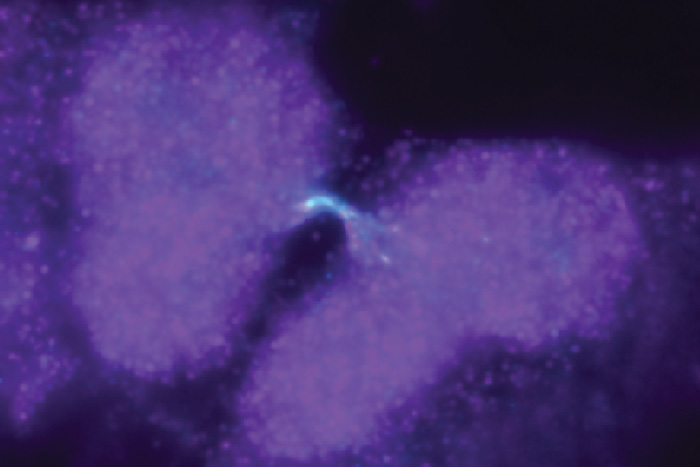
A chromosome bridge connects two dividing daughter cells. The image was captured using traditional brightfield microscopy.
The BFB cycle was first discovered in the late 1930s by the world-renowned geneticist and Nobel laureate Barbara McClintock, Ph.D., who conducted much of her work at Cold Spring Harbor Laboratory. While McClintock’s hypothesis of the BFB cycle was groundbreaking, the technology did not yet exist to pin down the molecular cause that triggers the cycle.
Now, as seen in the journal Nucleic Acids Research, an innovative study led by Manrose “Manny” Singh, a fifth-year student in the Osteopathic Medicine, D.O./Medical and Biological Sciences, Ph.D. (D.O./Ph.D.) program, builds upon McClintock’s work to provide a possible answer. The research was primarily conducted in the laboratory of Professor and Director of the Center for Cancer Research Dong Zhang, Ph.D., located on New York Tech’s Old Westbury campus—less than 10 miles from Cold Spring Harbor Laboratory.
To gain a clearer view of how the BFB cycle is initiated, the team, which also included researchers from Drexel University and Albert Einstein College of Medicine, recreated the kind of chromosome damage that potentially triggers the BFB cycle. They applied a modified CRISPR/Cas9, a popular gene-editing technology, to human cells and created roadblocks at the ends of many chromosomes to intentionally stall DNA duplication.
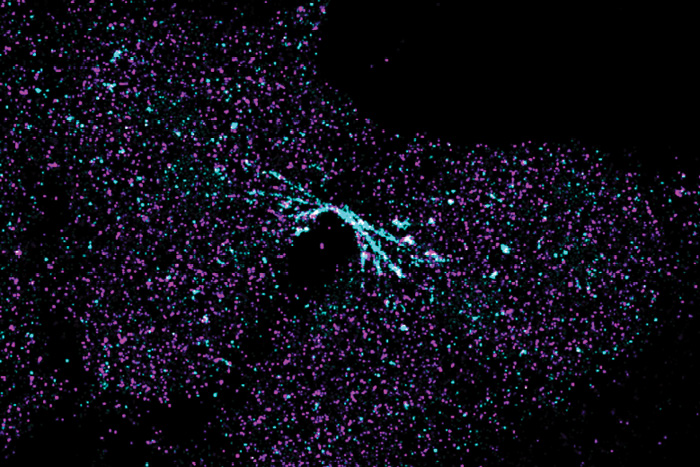
Image of a chromosome bridge captured using stochastic optical resolution microscopy (STORM), a type of super-resolution microscopy.
Then, they closely examined each stage of the BFB cycle and the molecular processes leading to fusion, bridging, and breakage. With the help of NYITCOM Senior Research Associate Lars Udo-Bellner and Associate Professor Randy Stout, Ph.D., they also captured super-resolution images of the chromosome bridges. Finally, they identified several new DNA repair pathways involved in fusion and bridges. Their findings will help to advance understanding of how tumors originate and inform the development of future cancer treatments.
“Our study not only honors Dr. Barbara McClintock’s pioneering work conducted more than 80 years ago but also pushes the boundaries of modern cancer research,” says Singh. “By using cutting-edge molecular and genetic tools to dissect the BFB cycle, we aim to help pave the way for new strategies that combat cancer.”
As a next step, Singh and other researchers in Zhang’s laboratory will further study the DNA sequence changes in the BFB cycle model to predict which cancers have undergone the BFB cycle during their development.
“I have a hunch that the ALT-positive cancers that we have been studying in the last 10 years are more likely to have undergone the BFB cycle”, says Zhang.
The ALT-positive cancers account for an estimated 10 to 15 percent of cancer cases, and include some of the deadliest kinds, including glioblastoma, osteosarcoma, and pancreatic neuroendocrine tumors. Zhang predicts that this new BFB cycle model will be widely used to investigate tumor metastasis, drug resistance, and how tumors form (tumorigenesis) Other NYITCOM authors on the Nucleic Acids Research paper include alumni Danny MacKenzie (D.O. ’22), Katherine Loomba (D.O. ’23), Sanket Desai (D.O. ’24), and Noelle Batista (D.O. ’24), as well as current student D.O./Ph.D. student Navjot Guru.










